
SOLUTION Lineweaver burk eadie hofstee hanes plot derivation from michaelis menten equation
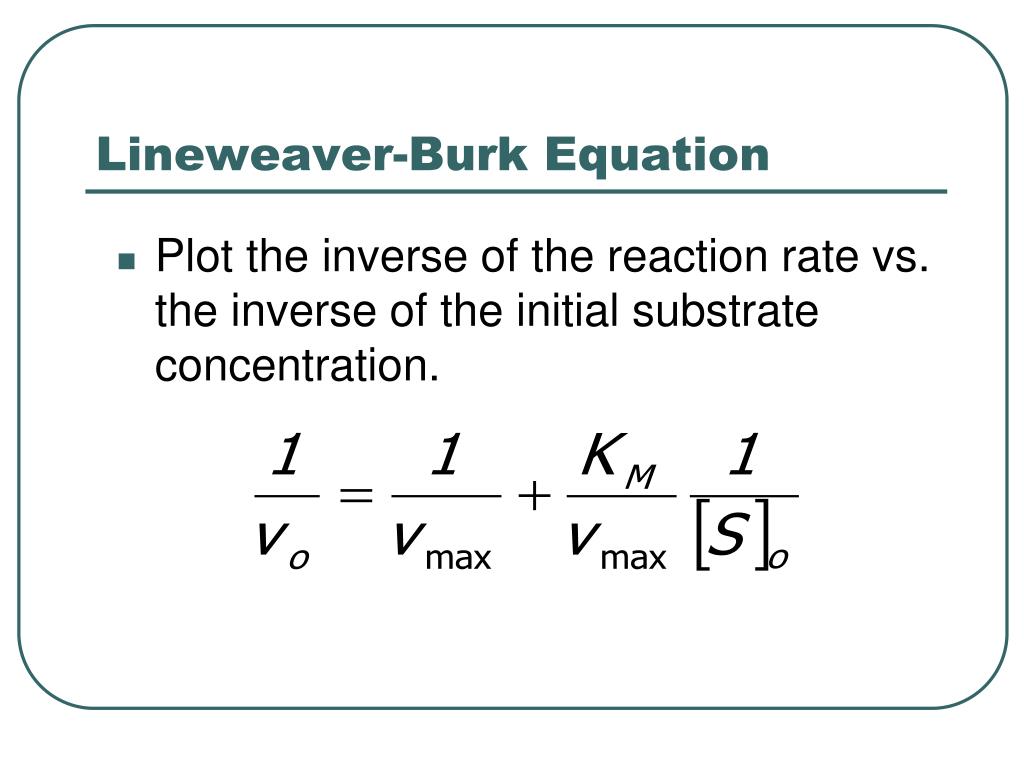
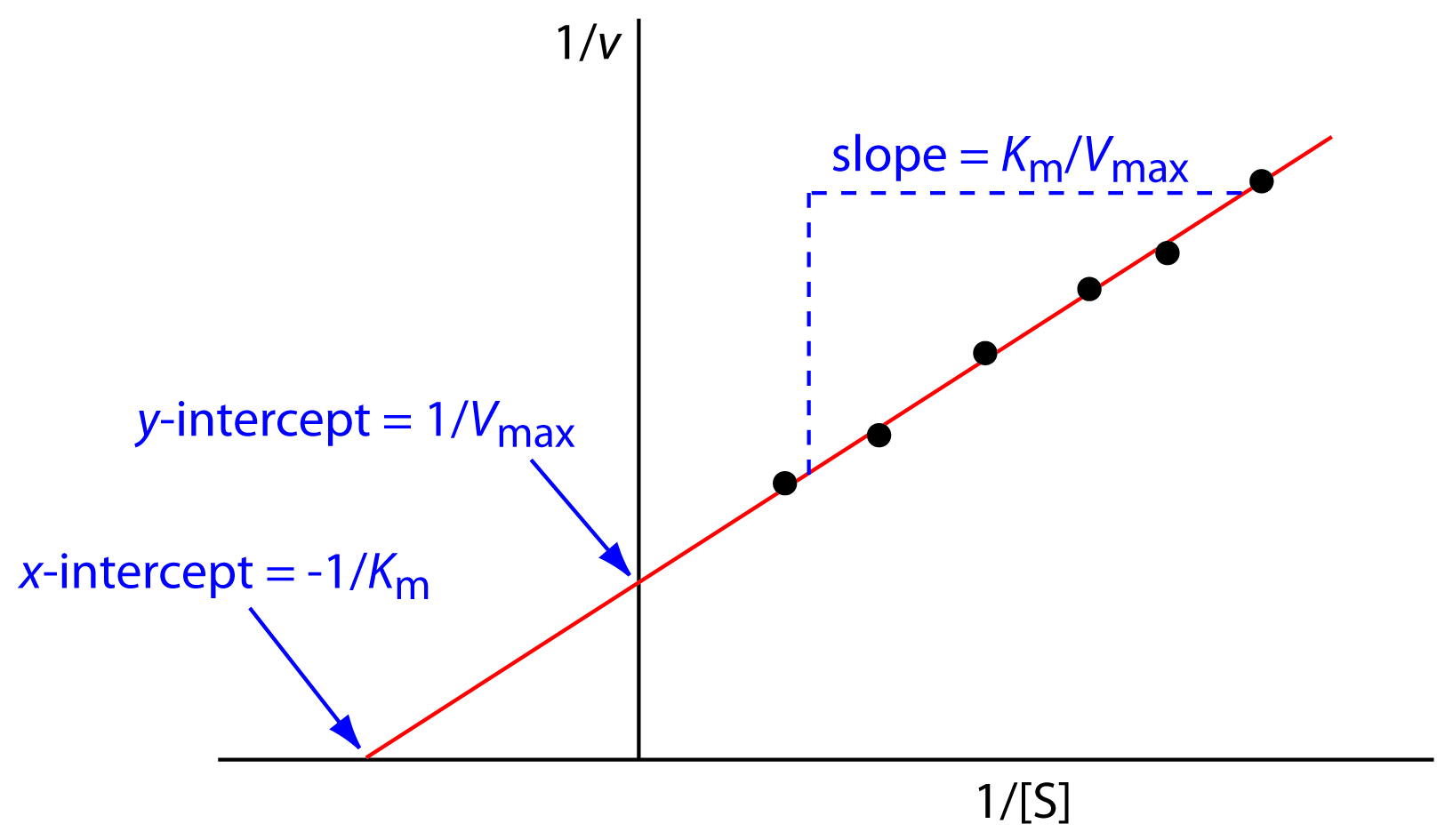
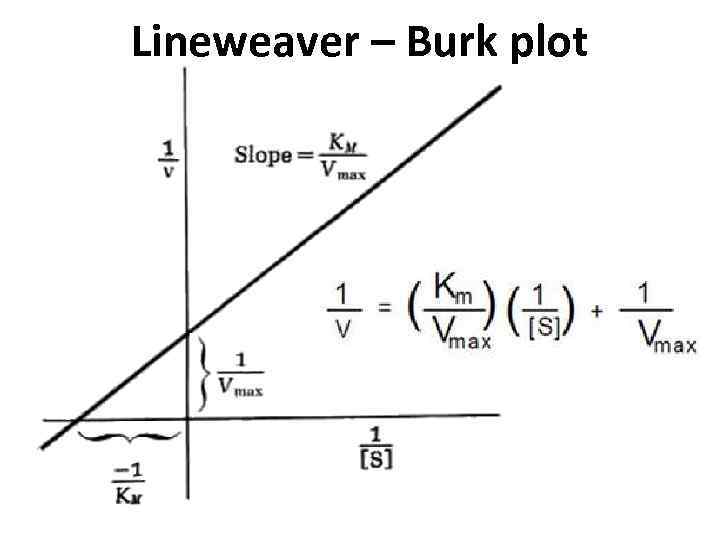
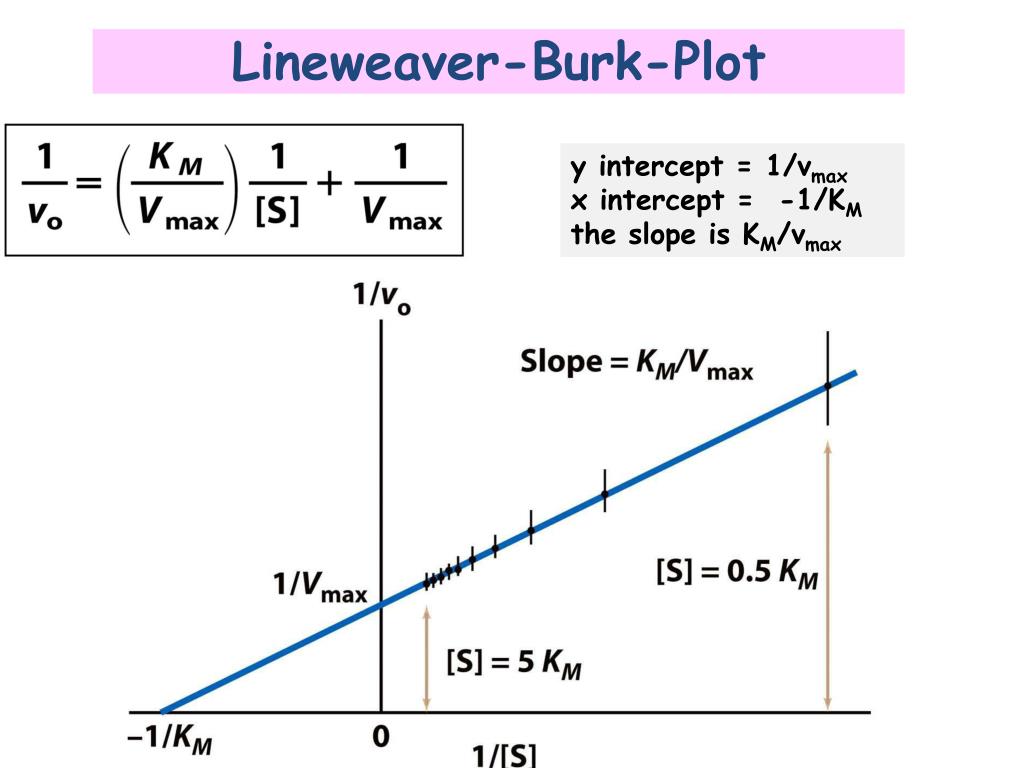
Lineweaver-Burk Plots. The Michaelis-Menten equation is useful in other ways, too. If we take its inverse, we get a new relationship. That's useful because it's really an expression for a straight line. If we plot 1/v against 1/[S], we get a straight line. The slope is K m /V max and the y intercept is 1/V max. Lineweaver-Burk plot gives a.
PPT Chemistry 232 PowerPoint Presentation, free download ID382916
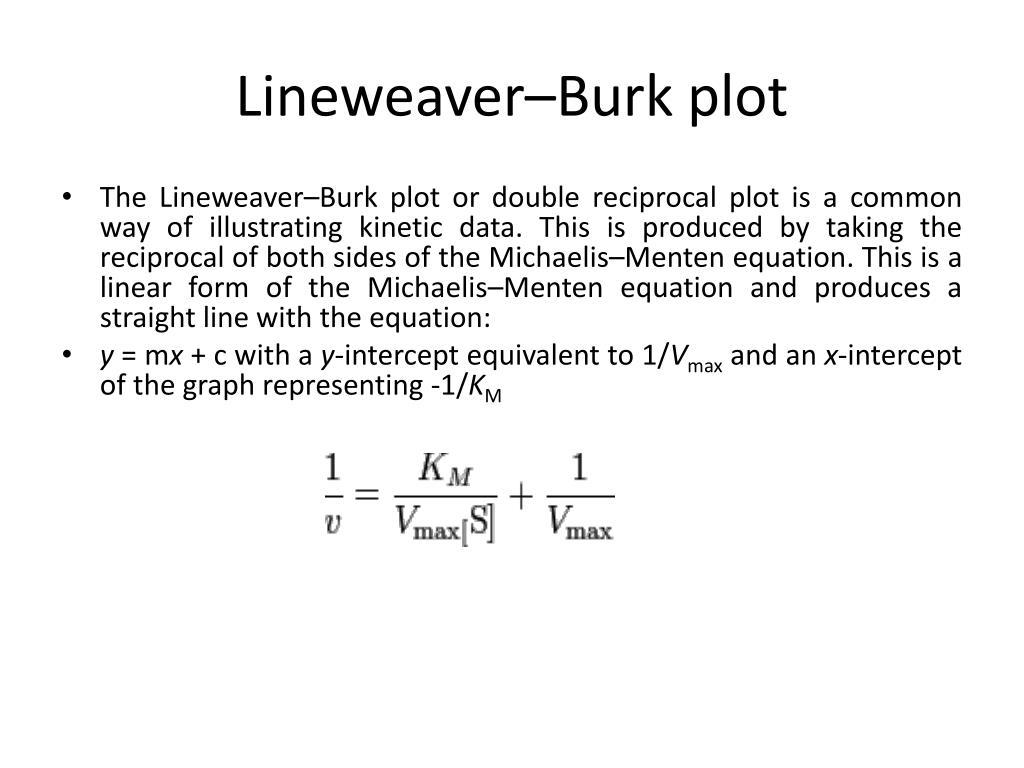
In biochemistry, the Lineweaver-Burk plot (or double reciprocal plot) is a graphical representation of the Michaelis-Menten equation of enzyme kinetics, described by Hans Lineweaver and Dean Burk in 1934. [1]
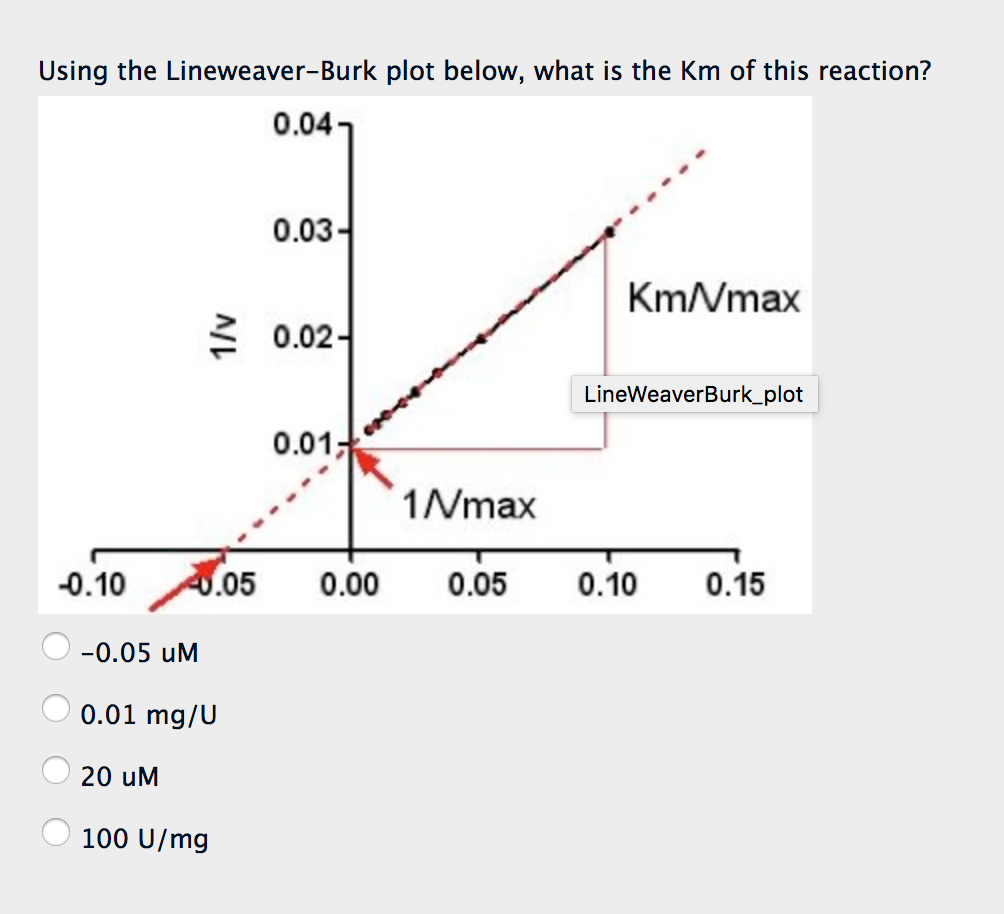
Solved Using the LineweaverBurk plot below, what is the Km
Lineweaver-Burk plot with data points derived from double-reciprocal transformation, but we'll superimpose a line based upon nonlinear regression analysis, so that it reflects the best possible estimates of K m and V max. A different secondary plot, such as Hanes-Woolf or Eadie-Scatchard, is just as easy to create with Prism.
PPT Chapter 6 Enzymes PowerPoint Presentation ID5143485
Lineweaver-Burk analysis is one method of linearizing substrate-velocity data so as to determine the kinetic constants Km and Vmax. One creates a secondary, reciprocal plot: 1/velocity vs. 1/ [substrate].

reaction mechanism Enzyme Given Km find substrate concentration at a certain
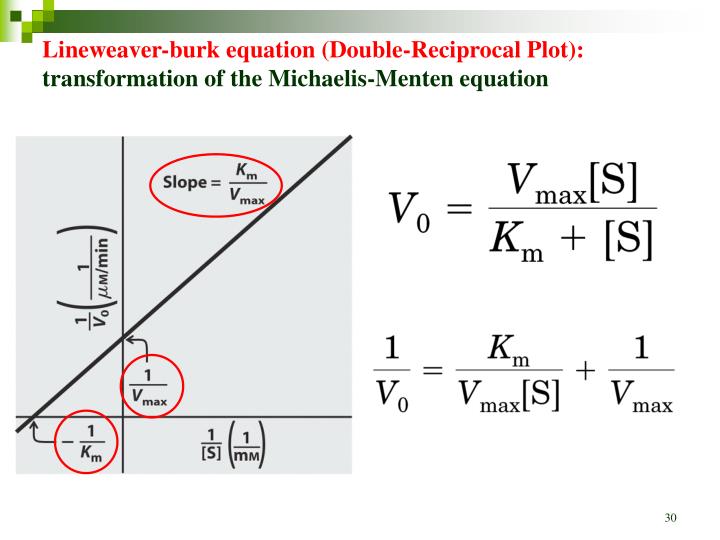
In Lineweaver Burk plots, the slope is equal to KM / Vmax, the x-intercept is equal to -1 / KM, and the y-intercept is equal to 1 / Vmax . The Lineweaver Burk plot is a graphical representation of enzyme kinetics.
13.2 Chemical Chemistry LibreTexts
affect the plots. A comparison between the two graphic representations direct is illustrated here with two "bad" data points (see Fig. 8.16, WWBH). •The same data points are plotted on adjacent Lineweaver-Burk in the left graph of this figure. Two features of the direct linear plot are immediately evident by comparison.

How to Calculate Km Sciencing
Another commonly-used plot in examining enzyme kinetics is the Lineweaver-Burk plot, in with the inverse of the reaction rate, \(1/r\), is plotted against the inverse of the substrate concentration \(1/\left[ \text{S} \right]\). Rearranging Equation \(\ref{Eq13.26}\),. Figure 13.12: Linweaver-Burk plot and regression equation for the data.
Вiochemistry of enzymes The overall Coenzyme role
The Lineweaver-Burk plot (or double reciprocal plot) is a graphical representation of the Lineweaver-Burk equation of enzyme kinetics, described by Hans Lineweaver and Dean Burk in 1934. This plot is a derivation of the Michaelis-Menten equation and is represented as: Table of Contents
PPT LAB 3 Enzyme PowerPoint Presentation, free download ID4526880
In biochemistry, the Lineweaver Burk Equation of energy kinetics results in a Lineweaver Burk Plot also known as a Double Reciprocal Plot. Therefore, users may wonder how to make a Lineweaver Burk plot in Excel. In this article, we demonstrate step-by-step procedures to make a Lineweaver Burk plot in Excel. Table of Contents Expand

LineweaverBurk plot to determine the Monod constants for... Download Scientific
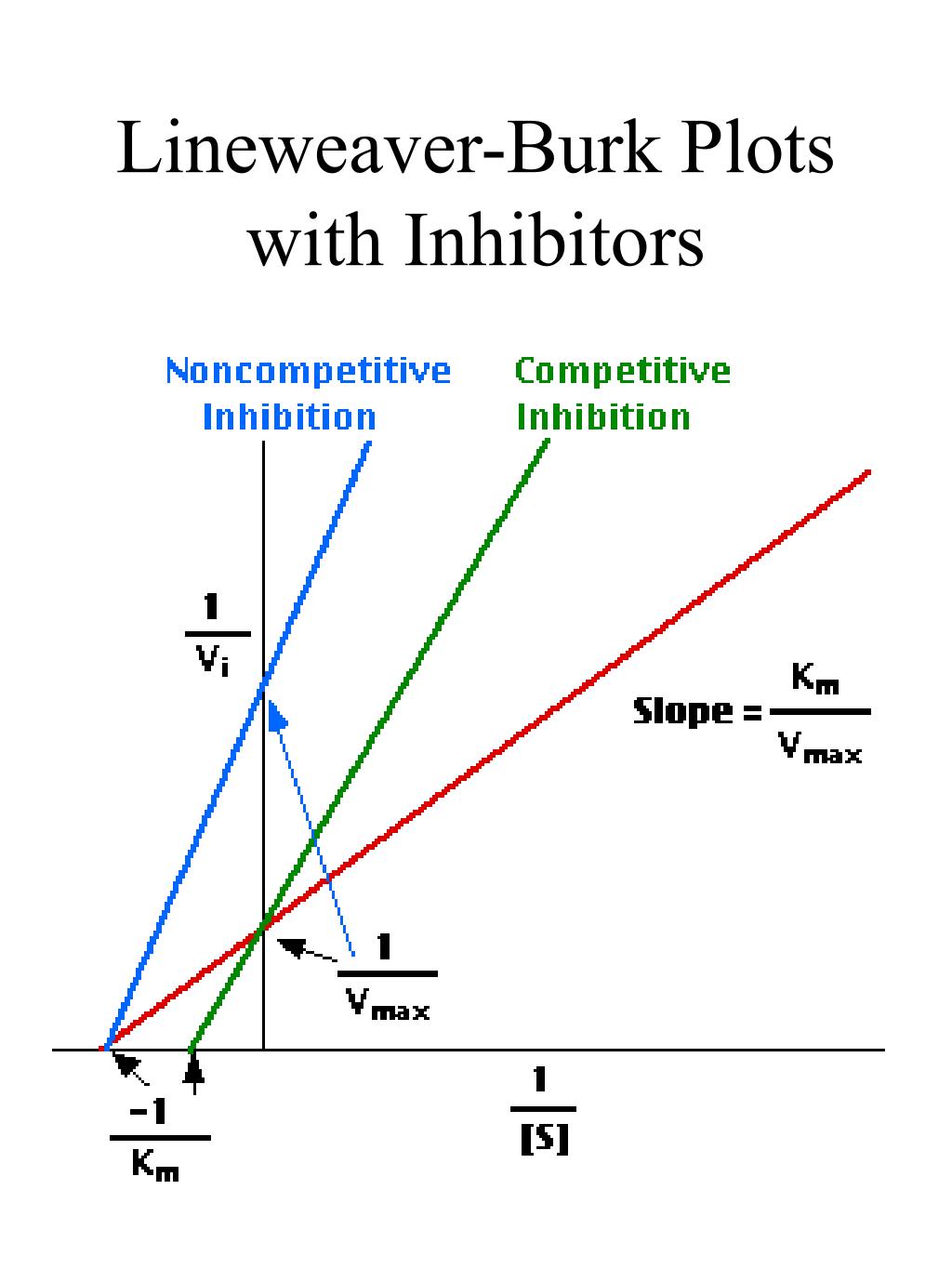
The double reciprocal plot (Lineweaver Burk plot) offers a great way to visualize the inhibition. In the presence of I, just Vm will decrease. Therefore, -1/Km, the x-intercept will stay the same, and \(1/V_m\) will get more positive.. An equation, shown in the diagram above can be derived which shows the effect of the noncompetitive.
PPT 513341 Biochemistry I Chapter 7 ENZYME PowerPoint Presentation ID4312460
Lineweaver-Burke (the "double reciprocal" plot) The Michaelis-Menten equation can be rearranged by taking the reciprocal, to yield: If X = 1/[S] and Y=1/V then this is a linear equation with a slope of K m /V max and a Y intercept of 1/V max; Figure 6.2.3: 1/S and1/V.

LineweaverBurk plots of reaction velocity versus substrate... Download Scientific Diagram
Lineweaver-Burke (the "double reciprocal" plot) The Michaelis-Menten equation can be rearranged by taking the reciprocal, to yield: If X = 1/[S] and Y=1/V then this is a linear equation with a slope of K m /V max and a Y intercept of 1/V max; Figure 6.2.3: 1/S and1/V

LineweaverBurk Plot Biochemistry, Enzyme Mcat
Lineweaver Burk plots show that the Vmax was calculated at 9 nmoles per mg per 30 min, or 1.3 nmoles per pineal per 30 min. From: Serotonin and Behavior, 1973 Add to Mendeley About this page Molecular Aspects of Inhibitor Interaction with PDE4 Siegfried B. Christensen,. Theodore J. Torphy, in Phosphodiesterase Inhibitors, 1996

LineweaverBurk Equation Theory and Derivation YouTube
Tthe Lineweaver-Burk plot (or double reciprocal plot) is a graphical representation of the Lineweaver-Burk equation of enzyme kinetics, described by Hans Lineweaver and Dean Burk in 1934 (Figure \(\PageIndex{2}\)).. Lineweaver-Burk plot of Michaelis-Menten kineitcs. The plot provides a useful graphical method for analysis of the.
PPT Lecture 4 PowerPoint Presentation, free download ID2832807
A. Data fit by nonlinear regression to a hyperbola. B. Data fit to a Lineweaver-Burk reciprocal plot. The gray line shows the fit obtained after omitting the point at the lowest substrate concentration. C. Data fit using the Eadie-Hofstee equation. In each figure, the equation and the resulting k cat and K m values are displayed.

(A) LineweaverBurk plot for the inhibition of eeAChE(A) and eqBChE (B)... Download Scientific
double reciprocal plot (e.g., Lineweaver-Burk plot discussed below;. Figure 13.12: Linweaver-Burk plot and regression equation for the data in Example 13.6.-diphenyl oxidase. The following data are for the oxidation of catechol (the substrate) to o-quinone by the enzyme o-diphenyl oxidase. The reaction is followed by monitoring the.